So these last few weeks I have been feeling quite lazy, the result of an epic 7 page post in Biology, show week, Math Day, Harry Potter 7, and 3 hours of Jazz Band rehearsal this morning. But, I figured I had better'd make a blog post on The Periodic table, The Conductivity Lab and an Article Review. Yawn, so here we go.......
PART 1. The Periodic Table. It has periods. And patterns.
 |
| Nicholas Mendeleev |
The second thing that he did differently was he occasionally ignored the order that atomic weights would suggest and switch adjacent elements like Cobalt and Nickel to group them better based on chemical properties. Eventually it would become apparent that he had (unknowingly, as atomic structure theories were still in the works) arranged the elements in order based on Atomic Number. Even later on, with the development of Quantum Mechanical Theories, it becomes apparent that each row of the table relates to the filling of the quantum shells of atoms.
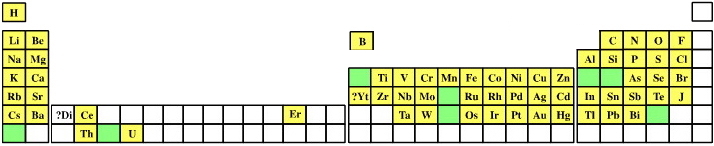
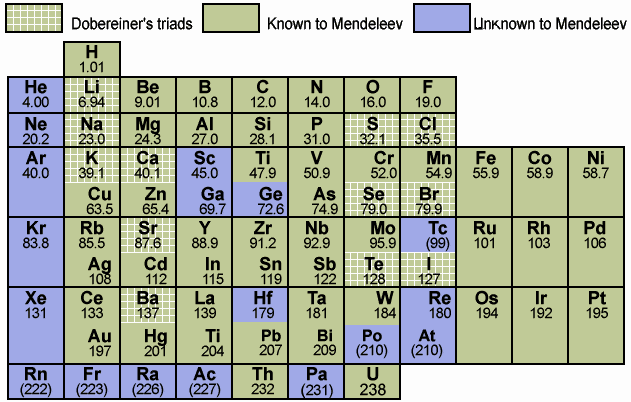 |
| Mendeleev's Known Table |
 |
| Mendeleev's Predicted Table |
 |
| Modern Table |
Groups:
Groups (or families) are are vertical columns in the periodic table and also the most important way of classifying the elements. In groups 1A-8A (Main Group Elements) the elements in the columns share certain properties (Number of valence electrons, atomic radius, etc...) all the way down the group. Each group has a trivial name ie: 1A is the Alkali Metals, 2A is the Alkaline Earth Metals, 3A the Boron Group, 4A is the Carbon group, 5A the Pnictogens, 6A the Chalcogens, 7A the Halogens, and 8A The noble gasses. In groups 3B-2B (the Transition Metals) groups are named by the uppermost element in the group. In the "A" groups the groups number is equal to the number of electrons in the valence electrons.
Periods:
Periods are the horizontal rows of elements. The only places in the table where periods are more important for classification than groups are the d-block (transition metals, groups 3B-2B) and the f-block (inner-transition elements, lanthanides and actinides). The main use for periods is determining the number of electron shells for each block. Modern quantum mechanics explains these periodic trends in properties in terms of electron shells. As atomic number increases, shells fill with electrons in approximately the order shown below. The filling of each shell corresponds to a row in the table.
- 1s (Groups 1A and 8A, Period One (hydrogen and helium))
- 2s (Groups 1A and 2A, Period 2 (Li,Be)) 2p (Groups 3A-8A, Period 2 (B,Ne))
- 3s 3p 3d
- 4s 4p 4d 4f
- 5s 5p 5d 5f
- 6s 6p 6d
- 7s 7p
- 8s
Blocks:
A block is a set of adjacent groups. The block names (s, p, d, f. and g) are derived from the quality of the spectroscopic lines of the associated atomic orbitals: sharp, principal, diffuse and fundamental, the rest being named in alphabetical order. Blocks are sometimes called families.The s-block is comprised of groups 1A-2A, the p-block is comprised of 3A-8A, the d-block is comprised of groups 3B-2B, the f-block is comprised of the lanthanides and actinides, the g-block is hypothetically comprised of the predicted 8th period.
Now there are some other ways of grouping elements (platinum group, noble metals, etc...), but no one really cares about those, mostly because they're are not really used in high school chemistry. So to end this first part of my 2nd quarter post I'll leave you with another image of the periodic table labeled with some of the things I've discussed. Phew.
No comments:
Post a Comment